Abstract
Cattle Egret (Bubulcus ibis) is one of the most well-known herons in Egypt. It is called the friend of the farmer because it benefits farmers and helps them get rid of insects and worms. It acts as a reservoir for many diseases. Few researchers have discussed the significance of parasitic diseases that affect this wild bird and may lead to mortalities among the population especially the importance of vital organs such as kidneys. Therefore, this study aimed to spotlight parasitic infection-affected herons in Egypt and consider the risks to this beneficial bird. During this study, 23 Bubulcus ibis were captured after their death from Abou Rewash Giza Governorate, Egypt, during the period from February to September (2022). Renicola species (spp.) and Apharyngostrigea spp. are two important digenean parasites that were recovered from the kidneys, and small intestine of the heron Cattle Egret (Bubulcus ibis) with an infection rate of (17.2%) and (11.8%) respectively. Histopathological techniques were used to assess tissue alterations while light microscopy and molecular assays were used to assess the parasites. The parasites’ morphological and morphometrical characteristics, as well as polymerase chain reaction and sequencing assays (mitochondrial sections), were investigated for the first time in Egypt. These parasites were given in-depth illustrations and drawings. The distinctive qualities of the two species were discussed. As the first record from Egypt, the nucleotide sequences discovered in this work have been uploaded into the GenBank database (accession numbers: OR021986 and OQ955829). Microscopically, the renal blood vessels had vasculitis, necrosis, and other degenerative alterations. Further research analyzing the health of various heron spp. and environmental deterioration can help to close information gaps about the interactions between parasites, their hosts, and environmental health.
Similar content being viewed by others
Introduction
Herons are crucial in the biological control of agricultural pests such as insects, mollusks, earthworms, and fish, and many of them serve as intermediate hosts for helminth parasites. The cattle egret (Bubulcus ibis) is a worldwide heron (family Ardeidae) that lives in the tropics, subtropics, and warm-temperate zones. The cattle egret (Bubulcus ibis) is common in Egypt1. In an agricultural country like Egypt, wild birds are widely distributed due to their capacity to adapt to large climatic fluctuations. Because man is frequently in touch with water, there is a risk of infection with avian parasites2. The order Ciconiiformes, which includes the ardeid birds, often known as egrets and herons, typically inhabit tropical and subtropical regions in wetlands, mudflats, ponds, lakes, and water reservoirs. Herons play a significant part in the biological management of agricultural pests such as insects, mollusks, earthworms, fish, reptiles, and rodents, many of which serve as intermediate hosts for the helminth parasite.
According to3, domestic birds can catch diseases from wild birds. For some possible zoonotic helminth spp., some ardeids can serve as the sole hosts, and they may also be involved in the spread of digenetic trematodes from freshwater fishes4. The impact of parasite infection may be influenced by a variety of intrinsic and external factors. Although it is widely accepted that host characteristics such as age, sex5, and sexual maturity6 influence parasite community structures and susceptibility to parasitism, studies describing these associations are limited7. Other infections (viral, bacterial, or fungal), immunosuppression, and environmental changes, in addition to host susceptibility, may alter parasite number, diversity, and distribution8,9,10.
Flukes from various trematode genera, including Renicolidae can infect the kidneys of birds. Renicolidae, a family of small digeneans that inhabits the renal tubules and ureters of herons, has been described11. Parasitism may thus impact individual organs and alter the host's physiology and ecological behavior. Data on the presence of renicolids and their effects on the kidney in Ardea goliath are particularly inadequate12,13. Other seabird species have shown severe kidney abnormalities in studies14. Renicolids have five life stages: eggs, miracidium, sporocysts, cercaria, metacercaria, and adult15. After the primary intermediate host (marine gastropods) ingests the miracidium inside the egg, the sporocysts grow. The free larval form of the parasite, cercariae, enters the second intermediate host, marine fishes, where it transforms into metacercariae16.
Concerning the life cycle of Apharyngostrigea spp., the eggs obtained from infected herons were developed into miracidia and then entered freshwater intermediate hosts (planorbid snails) from which emerge distinctive furcocercous cercariae forming metacercariae in fish or amphibians17. Only a few samples from the United States have had their mitochondrial (mt) DNA sequenced18, whereas sequences from samples from other locations are only slowly evolving nuclear rDNA markers. Effectively, this circumstance has made it impossible to analyze the geographic distribution of Apharyngostrigea spp. using molecular methods. In line with17, two species of Apharyngostrigea that were sampled from a variety of intermediate and final hosts in the Americas and Africa were examined morphologically and genetically.
The aim of the current study is an updated spotlight on the existence of trematodes infecting Cattle Egret (Bubulcus ibis) in Giza, Egypt, with a focus on their first morpho-molecular identification in Egypt and histopathological findings.
Material and methods
Sample collection
During this study, 23 Bubulcus ibis were captured after their death from Abou Rewash Giza Governorate, Egypt during the period from February to September 2022 and brought to the laboratory and immediately dissected, Buccal cavity, trachea, intestine, and kidneys were examined carefully for any parasitic infestation. The collected trematodes were fixed in 10 ml Formalin, 50 ml Ethanol, 5 ml Glacial acetic acid, and 45 ml distilled water then cleared in lactophenol or stained with acetocarmine. The specimens are measured by using a light microscope with a divided ocular lens and all measurements are done in millimeters13,19,20. The identification of Renicola spp. was confirmed and identified according to21.
Molecular analysis (DNA extraction, PCR, and sequencing)
According to7 and17, trematodes were eliminated from the kidneys, and DNA was extracted using a commercial kit (DNA Blood and Tissue kit, QIAGEN®, Valencia, CA). The primers 5′ TTTTTTGGGCATCCTGAGGTTTAT 3′ and 5′ TAAAGAAAGAACATAATGAAAATG 3′ were used to conduct the amplification of the COXI (cytochrome C oxidase, subunit I) areas for Renicola spp. Primer was 5′ ATTAACCCTCACTAAATTATCACGTTTAGGATCATAAG 3′ and 5′ TAATACGACTCACTATAGCATGATACACTAAATTTACTCGATC 3′ in the case of Apharyngostrigea spp. The PCR conditions were 94 °C for 2 min, 35 cycles of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 1 min, followed by a 10-min extension at 72 °C22. By electrophoresis on a 2% agarose gel in a TBE buffer containing ethidium bromide (0.5 g/mL), the PCR amplicons were identified and thereafter visible under UV light. The PCR amplicons were purified with the PCR DNA and Gel Band Purification Kit (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and quantified with an Invitrogen Life Technologies Qubit® Fluorometer (Eugene, OR, USA). The original forward and reverse primers used in the initial PCR experiment have been utilized to sequence the PCR amplicons using a BigDye® Terminator Cycle Sequencing Kit in an Applied Biosystems Genetic Analyzer sequencer. The assembled sequences were examined and aligned using the BLAST tool found at (https://blast.ncbi.nlm.nih.gov/Blast.cgi) against other pertinent sequences that had been deposited in GenBank23. The GenBank database has received the nucleotide sequences obtained in this investigation. The GenBank database received the edited COXI sequences in order to assign accession numbers. The Neighbor-Likelihood and Maximum Likelihood methods in MEGA (version 11) were used to create the phylogenetic trees based on nucleotide sequences24. The neighbor-joining and Maximum Likelihood phylogenetic analysis were used to determine the evolutionary relationships between the two present sequences of Renicola and Apharyngostrigea spp. versus correlated sequences.
Pathological examination
According to the25 procedure, samples were obtained from each infected kidney tissue of several birds preserved in 10% buffered formalin. Tissues were fixed in formalin for at least 24 h. Processed samples were cut with semi-automated microtome at a thickness of 5 µm. For histological investigation using light microscopy, samples were prepared using the traditional paraffin embedding technique, sectioned at 4–5 µm, and stained with hematoxylin and eosin stain. Using an Olympus CX41 microscope, the affected organs were examined and captured on camera.
Statistical analysis
Using SPSS 27 (IBM, NY, USA), The mean, maximum, and minimum values associated with every character, as well as the length/width ratios for the suckers and the body, were determined by a statistical description26,27.
Ethical approval
All experimental protocols were approved by the guidelines of the ethical committee (Institutional Animal Care and Use Committee), Faculty of Veterinary Medicine, Cairo University (Vet CU. IACUC-08072023699).
Results
Morphological characteristics
The present study revealed two digenetic trematodes collected from Cattle Egret (Bubulcus ibis). The incidence of infected birds was 17.2% and 11.8% with Renicola spp. and Apharyngostrigea spp. respectively.
The group of specimens (15 worms) obtained from the kidneys of the examined Cattle Egret (Bubulcus ibis) proved to belong to the family Renicolidae, genus Renicola and had the following morphological descriptions (Figs. 1, 2 and 3). The body is pyriform in shape, widest anteriorly and tapering posteriorly to produce a caudal prolongation. The length ranges from 4.0 to 5.0 (mean 4.7) mm, and the maximum breadth ranges from 3.1 to 3.5 (mean 3.2) mm. The spines cover the cuticle. The ventral sucker has a diameter of 0.1–0.2 mm. The oral sucker is big and subterminal, measuring 0.5–0.8 × 0.6–0.9 mm (mean 0.67 × 0.81) in size. The sucker's lumen is funnel-shaped and opens directly into the elongated pharynx, which measures 0.13–0.27 × 0.15–0.17 mm (mean 0.22 × 0.16). The wide caeca divides right after the pharynx and extends back to the back end of the body, almost entering the caudal extremity. The division of the intestinal caeca is disguised by the anterior coils of the uterus. The right testes are covering the ventral sucker, and the two testes look like two huge masses. The ovary is heavily branched and extends diagonally from the right testes to the left side of the body, in front and behind the ventral sucker. It seems to be made up of several distinct, large follicles. In the linear distribution, vitellaria takes the shape of coarse follicles and covers two lateral fields of the body. The uterus, which coils to take up the majority of the worm’s body, is large and developed. The mature egg has a thin, light brown, operculated shell and is tiny. It weighs 0.032 × 0.012–0.037–0.014 (mean 0.034 × 0.013) mm and has a miracidium within.
(A) Fresh specimens of Kidney of Cattle Egret (Bubulcus ibis) infected kidney (IK- arrows) with the Renicola parasitic cyst and normal Kidney (NK) are observed macroscopically. (B) Fresh specimens of Renicola spp. from freshly dead Cattle Egrets. (C) Renicola spp. Eggs. IK infected kidney; NK normal kidney.
Other specimens (11 worms) were also collected from the kidneys and belonged to the same genus but with some morphometrical differences (Figs. 2, 3). The oral sucker is larger and spherical in shape, it measures 0.87–1.1 (mean 0.98) mm, in diameter. The pharynx is round in shape and present. At the level of the oral sucker. It measures 0.22–0.27 (mean 0.25) in diameter, Ventral sucker is rounded and lies between the testes, behind the vitelline duct, it measures 0.09–0.13 (mean 0.11) mm. in diameter. The two testes lie side by side in a V-shaped manner immediately behind the transverse vitelline duct. The branched ovary occupies a narrow area at the right of the midbody and crosses the transverse vitelline duct. Vitellaria is in the form of coarse follicles that occupy the middle third of the two lateral fields. The egg is ovoid, light in color, thick-shelled shelled and operculated, it contains a miracidium and measures 0.026 × 0.012–0.019 (mean 0.037 × 0.016) mm.
On the other hand, there were (10 worms) collected from the intéstine and proved to belong family Strigeidae, genus Apharyngostrigea. and had the following morphological characteristics (Figs. 4, 5). The body of the parasite is divided into a forebody ending at the posterior margin of the tribocytic organ and a cylindrical hind body which is larger than the forebody. The parasite is 2.2–4.2 (mean 3.6) mm, in length. The ratio of the fore/hind body is 1:2. The body is covered with minute spines. Suckers are well developed, oval in shape and ventral larger than oral measuring 0.30–0.35 × 0.28–0.38 mean (0.33 × 0.29) mm. Pharynx absent. Proteolytic gland in the intersegmental region. A small kidney-shaped ovary lies in the midline of the hind body. It occurs at a distance of 1.09 mm. from the posterior end of the tribocytic organ and measures about 0.13–0.15 × 0.24–0.32 (mean 0.135 × 0.29) mm. The testes are two multilobulated, and tandem in position. Vitellaria, are large follicles that occupy both fore and hind bodies. Eggs are oval in shape, thin shells Yellowish in color and measure 0.089–0.115 × 0.64–0.077 (mean 0.10 × 0.073) mm. It contains an ill-developed embryo.
Molecular identification
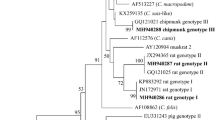
Interestingly, two main clades were identified by the phylogenetic analysis of COXI gene sequences. The amplified products of COXI had 434 bp and 428 from Renicola spp. and Apharyngostrigea spp., respectively. The sequence of COXI locus of our Renicola spp. (accession number: OR021986) showed higher nucleotide similarity 100%, with accession number: (ON652712; ON652665) and 99.57% with accession number: (ON652700; ON652648). The lower nucleotide similarity was with R. buchanani (87.37%; accession number: KF512572), R. lari (86.02%; accession numbers: KU563725) and R. pinguis (84.54%; accession numbers: KU563724). In the case of Apharyngostrigea spp., the sequence of COXI locus (accession number: OQ955829) showed higher nucleotide similarity 100%, with accession number: (MH777789) and 98.73% with accession number: (MH777791). The lower nucleotide similarity was with A. cornu (87.95%; accession number: JF769451), A. pipientis (86.79%; accession numbers: MT943779) and Australapatemon spp. (83.9%; accession numbers: MH369768). In the phylogenetic trees (Figs. 6, 7) the Bubulcus ibis from this study clustered with Renicola spp. and Apharyngostrigea spp., as revealed by the analyses of COXI locus. The Bubulcus ibis digenean parasites strain formed a separated branch from the other Renicolidae spp. and Strigeidae spp.
Renicola spp. phylogenetic analysis using COXI regions. The Maximum Likelihood method was used to build the tree. The number of nucleotide substitutions per site is shown by the scale bar at the base of the tree. The GenBank accession numbers of the strains were provided, and the sequences from this investigation are identified by a filled circle. Outgroups included the Cichlidogyrus spp., Dadaytrema minimum, Glypthelmins facioi and Paragonimus kellicotti.
Apharyngostrigea spp. phylogenetic study based on COXI regions. The Neighbor-Joining method was used to build the tree. The number of nucleotide substitutions per site is shown by the scale bar at the base of the tree. The GenBank accession numbers of the strains were provided, and the sequences from this investigation are identified by a filled circle. Outgroups included the Australapatemon spp., Neodiplostomum spp. and Apatemon spp.
Pathological findings
Grossly the infected kidneys appeared enlarged and pale in color, with multiple dark nodules on their surface. These nodules contained two or more adult parasites and were surrounded by white yellowish caseated material and encapsulated by grey to the black thick well-defined capsule. Histopathological examination revealed the presence of considerable numbers of Renicola spp. (Fig. 8) in the renal parenchyma in the cortex and near the renal pelvis. The adult trematodes varied in size and contained great numbers of eggs of different sizes and shapes; they were ovoid with a basophilic stained curved body.
Macroscopical and histopathological picture of Renicola spp. infection in Cattle egret kidney. (A) Microphotograph of the heron kidney showing the presence of paired trematodes inside the parasitic cyst (H&E, 40x). (B) Microphotograph showing severe cystic dilatation, swelling and vacuolation of the renal tubule (H&E, 200x). (C) Kidney showing the presence of multiple numbers of eggs making pressure atrophy on renal tubules (H&E, 300x). (D) Microphotograph showing vasculitis of the renal blood vessel with thickening of its wall, perivascular edema and proliferation of fibroblasts. (E) Microphotograph showing diffuse degenerative changes of renal tubules and glomeruli with infiltration of inflammatory cells, note the cystic dilatation of the renal tubules in the center (H&E, 100x). (F) Microphotograph of the non-infected heron kidney showing the normal histological architecture of renal parenchyma (H&E, 200x).
The parasites were surrounded by an inner cuboidal epithelial lining and outer delicate connective tissue fibers with few inflammatory cells, mainly macrophages and lymphocytes. The renal tubules near the parasitic nodules suffered from pressure atrophy. Some renal tubules showed granulation and vacuolation of their cytoplasm (Fig. 8), whereas others suffered from coagulative necrosis. Regenerating renal tubules were noticed in most cases. The intratubular stroma was densely infiltrated with inflammatory cells, mainly macrophages and lymphocytes. Free eggs of the parasites were seen in between the renal tubules. The renal blood vessels were congested with focal areas of hemorrhages near some blood vessels. Perivascular edema and aggregation of parasitic eggs were observed in most cases. Numerous eggs in the blood vessels suggest that they could be moved to other organs or stall in the blood vessels as emboli, causing significant problems in these birds.
Discussion
The cattle egret is a valuable bird among cattle farmers due to its presumed role as a biocontrol of livestock parasites such as ticks and flies. In Egypt, the cattle egret (Bubulcus ibis) is an important bird and serves as a disease reservoir. Few researchers have discussed its significant contribution to disease transmission28. Renicolid trematodes are parasitizing tissues of kidneys and ureters of birds feeding on bivalves and fishes29. Worms although hermaphrodite normally live in pairs within dilated kidney tubules lined by cuboidal cells13. In this study, the total incidence of infected birds with Renicola spp. was 17.2%; this result disagreed with30 who recorded an infestation rate of 43.75% in Assiut. On the other side13, recorded an infestation rate of 12.4% in Iraq. The differences in the prevalence of Renicola spp. may be due to the availability of first (mainly crustaceans or mollusks) and second (fish) intermediate hosts.
The first trematode was of Renicola spp. morphologically resembles R. goliath but differ in the shape of the testes which appear as two large masses overlapping the ventral sucker while, in Renicola spp. it is lobed and lies side by side behind the ventral sucker. In the present specimens, the ovary appears highly branched and diagonally occupies a wide area with the testes. It appears as a deeply lobulated mass lying in front of the ventral sucker Renicola. The most outstanding feature that characterizes the first group of Renicola in comparison with Renicola spp. is its distribution of vitelline follicles. Vitellaria in the form of coarse follicles are in a linear distribution and occupy the two lateral fields of third third-fourth of the body, while, in the Renicola spp; it is distributed in the median and lateral fields of the intestinal caeca. Moreover, the other Renicola specimens morphologically resemble R. ardeolae13 but differ in the shape of the testes, it appears as a V-shaped form around the ventral sucker. This variation in the form of the testes was not described before. Where it appears as two branched testes and tandem in position; one anterior and one posterior to the ventral sucker in R. ardeolae30.
Vitellaria is in the form of coarse follicles in a pyramidal mass that occupy the lateral fields of the whole length of the intestinal caeca in R. ardeolae30. The present specimens, the vitelline follicles, are in a linear form and occupy the middle fourth of the two lateral fields. The specific name is derived from the locality and is considered a new species, of the third trematode species of Apharyngostrigea spp. differ from the homologous species, previously mentioned by30 where they described A. ibis.
In the present study, the morphological description of Apharyngostrigea spp. agreed with what was previously described by3,20 However, the present species differed from Apharyngostrigea ibis31 in the body length, length proportion between hind- and forebody and sizes of various organs, especially the proteolytic gland. Moreover30, described A. magna from Ardea goliath. Generally, in many helminths, the body surface plays an important role in integrating the worm's physiology with its micro-environments via nutrient assimilation, regulation of internal chemical pools by selective absorption or secretion mechanisms and chemosensory activity32. The results of the current investigation demonstrated that contact receptors covered the body surface of the Apharyngostrigea. Many different varieties of tegumental papillae may aid in feeding by expanding the absorption surface area. It also has a significant impact on the perception and selection of the materials to be ingested3. It is suggested that Apharyngostrigea external surface of the tegument might have other properties besides being absorptive; such as immunological protection and the maintenance of the worms in their habitat and assistance in retaining the position of the parasite within its host and mechanical stimulation of the host tissue for feeding purposes.
The present species is more related to Apharyngostrigea ibis (A. ibis) than A. magna where the major differences appear in the distribution of vitellaria in the hind body only in A. ibis, while it is present in the fore and hind body in the present specimens. The ovary takes the kidney in the present specimens but, it is rounded in A. ibis. Moreover, the distance between the tribocytic organ and ovary is large about 1.09 mm in the present specimens, while, it measures 0.21 mm. in A. ibis and ranges from 2.5 to 3.0 mm. in A. magna. The present specimens can be differentiated from A. magna30, in its three times smaller, Ratio of fore/hind body is 1.2 (1:3 in A magna) as well as the presence of suckers and characteristic proteolytic glands in the forebody of the specimens under discussion. Also, there is a difference in the locality of the herons3. These differences are enough to create the described as a new Spp. of Apharyngostrigea.
The phylogenetic analysis enabled us to identify the Renicolidae spp. and Strigeidae spp. found in this study. The sequence of COXI locus of Renicola spp. showed higher nucleotide similarity with33. The lower nucleotide similarity was with R. buchanani34, R. lari7 and R. pinguis16. Apharyngostrigea species were examined in this study as well as those with compatible COXI sequences in17,18. To confirm current and historical records of Apharyngostrigea spp. in South America, it would therefore be useful to get comparison sequences from Australia. Additional sequencing could also clarify historical records in North America35 and Africa20. According to36, there is little change in mitochondrial COXI between Apharyngostrigea pipientis and A. cornu. A. cornu’s potential existence in North America is supported analogously by the widespread distribution of A. pipientis37.
Our discussion was based on the statements of12,15, due to the limited availability of material on Renicola spp. and its impact on Bubulcus ibis tissues. In the current investigation, the kidneys displayed inflammatory cells in the intertubular stroma as well as degenerative alterations, including the necrosis of several renal tubules. These changes can be explained by the existence of circulating antigen toxins and immune complexes, which are potent initiators of inflammatory reactions because of parasitic infestation. The degenerative alterations and necrosis seen in this study are caused by circulating toxins14.
The endothelial damage caused by Renicola spp. eggs that were seen in the current study may have been directly caused by vasculitis, or they may have been caused indirectly by the impact of numerous toxic parasite products on the arteries wall. According to38, immune complexes that have accumulated in the vessel wall and caused damage may also be responsible for vasculitis. The hemorrhages and perivascular edema seen in this study can be explained by the involvement of the intima and endothelial lining of the blood arteries, which leads to vascular leakage and outflow of blood plasma and erythrocytes15.
Conclusion
One of the most well-known herons in Egypt is the cattle egret. It is known as the farmer's friend since it benefits farmers and aids in their control of worms and insects. It serves as a disease reservoir. Therefore, Continuous monitoring programs are crucial tools for increasing our awareness of the abundance, variety, and ecological characteristics associated with several heron species. The findings of this study showed a higher incidence of Renicola spp. than Apharyngostrigea spp., as well as a strong correlation with renal impairment in Cattle egret. Through morphological and molecular biological analysis, the parasite was identified as Renicola spp. and Apharyngostrigea spp. Parasites are found in practically every population and interact with the host in a variety of ways. Further research analyzing the health of various heron species and environmental deterioration can help to close information gaps about the interactions between parasites, their hosts, and environmental health.
Data availability
All the authors declare that all the data supporting the results reported in our article were included in this article. The datasets generated or analyzed during the current study are available in the GENE BANK repository, accession numbers: [OR021986 and OQ955829].
References
Hering, J., Fischer, S., Geiter, O., Wobker, J. & Siegel, S. Breeding colonies of gull-billed tern Gelochelidon nilotica and little tern Sternula albifrons on Lake Nasser, Egypt. Sandgrouse 43, 194–215 (2021).
Mahdy, O. A. & El-Ghaysh, A. Spiruroid parasites of buff backed heron (Ardeola ibis ibis) with a new species of Cordonema and Microtetrameres together with a key to the genera of Acuariidae (Seurat, 1915). J. Egypt. Ger. Soc. Zool. 27, 73–90 (1998).
Ammar, K. N. A. E. W. Surface topography of two trematodes parasites infecting grey heron Ardea cinerea Jouyi (Aves, Ciconiiformes) in Qena, Egypt. J. Egypt. Soc. Parasitol. 45(1), 143–152. https://doi.org/10.12816/0010860 (2015).
Essa, F. K. A. Role of fish eating bird Ibis-ibis in transmitting some parasitic larval stages to freshwater Oreochromis niloticus fish. Egypt. J. Agric. Res. 78(1), 105–116 (2000).
Gutiérrez, J. S., Rakhimberdiev, E., Piersma, T. & Thieltges, D. W. Migration and parasitism: Habitat use, not migration distance, influences helminth species richness in Charadriiform birds. J. Biogeogr. 44(5), 1137–1147. https://doi.org/10.1111/jbi.12956 (2017).
Thieltges, D. W., Hussel, B. & Baekgaard, H. Endoparasites in common eiders Somateria mollissima from birds killed by an oil spill in the northern Wadden Sea. J. Sea Res. 55(4), 301–308. https://doi.org/10.1016/j.seares.2005.12.001 (2006).
De Matos, A. M. R. N. D. et al. Molecular identification and histological aspects of Renicola sloanei (Digenea: Renicolidae) in Puffinus puffinus (Procellariiformes): A first record. Rev. Bras. Parasitol. Vet. 28, 367–375. https://doi.org/10.1590/S1984-29612019025 (2019).
Lafferty, K. D. Parasites in marine food webs. Bull. Mar. Sci. 89, 123–134. https://doi.org/10.5343/bms.2011.1124 (2013).
Falkenberg, J. M. et al. Gill parasites of fish and their relation to host and environmental factors in two estuaries in northeastern Brazil. Aquat. Ecol. 53, 109–118. https://doi.org/10.1007/s10452-019-09676-6 (2019).
Mahdy, O. A., Abdelsalam, M. & Salem, M. A. Molecular characterization and immunological approaches associated with yellow grub trematode (Clinostomid) infecting Nile Tilapia. Aquac. Res. https://doi.org/10.1155/2023/5579508 (2023).
Gibson, D. I. Family Renicolidae Dollfus. In Keys to the Trematoda Vol. 3 (eds Bray, R. A. et al.) 591–594 (CABI, CAB International, 2008). https://doi.org/10.1079/9780851995885.0000.
Mahdy, O. A. & Shaheed, I. B. Histopathological study on the effect of Renicola heroni on the kidneys of giant heron Ardea goliath. Helminthologia 38, 81–83 (2001).
Mohammad, Z. A. A. & Awad, A. H. H. New record of kidney trematode Renicola sp. in Squoacco Heron Ardeola ralloides, Cattle Egret Bubulcus ibis and White Pelican Pelecanus oncrolalus in Thi-Qar governorate Southern Iraq. J. Basrah Res. Sci. 40(3), 101–108 (2014).
Jerdy, H. et al. First report of kidney lesions due to Renicola sp. (Digenea: Trematoda) in free-living Magellanic Penguins (Spheniscus magellanicus Forster, 1781) found on the coast of Brazil. J. Parasitol. 102(6), 650–652. https://doi.org/10.1645/16-29 (2016).
De Matos, A. M. R. N. et al. Renicolidae infection in Manx shearwater (Puffinus puffinus): Is parasitism implicated on renal lesions?. Parasitol. Res. 120(4), 1311–1320. https://doi.org/10.1007/s00436-020-06959-y (2021).
Heneberg, P., Sitko, J., Bizos, J. & Horne, E. C. Central European parasitic flatworms of the family Renicolidae Dollfus, 1939 (Trematoda: Plagiorchiida): Molecular and comparative morphological analysis rejects the synonymization of Renicola pinguis complex suggested by Odening. Parasitology 143(12), 1592–1604. https://doi.org/10.1017/S0031182016000895 (2016).
Locke, S. A. et al. Intercontinental distributions, phylogenetic position and life cycles of species of Apharyngostrigea (Digenea, Diplostomoidea) illuminated with morphological, experimental, molecular and genomic data. Int. J. Parasitol. 51(8), 667–683. https://doi.org/10.1093/zoolinnean/zlab114 (2021).
López-Hernández, D. et al. Molecular, morphological and experimental-infection studies of cercariae of five species in the superfamily Diplostomoidea (Trematoda: Digenea) infecting Biomphalaria straminea (Mollusca: Planorbidae) in Brazil. Acta Trop. https://doi.org/10.1016/j.actatropica.2019.105082105082 (2019).
Mahdy, O. A., Mahmoud, M. A. & Abdelsalam, M. Morphological characterization and histopathological alterations of homologs Heterophyid metacercarial coinfection in farmed mullets and experimental infected pigeons. Aquac. Int. 28, 2491–2504 (2020).
Mahdy, O. A. et al. Epidemiological study of fish-borne zoonotic trematodes infecting Nile tilapia with first molecular characterization of two heterophyid flukes. Aquac. Res. 52(9), 4475–4488 (2021).
Mahdy, O. A. Giant heron (Ardea goliath) in Giza Egypt. Annu. Vet. Congr. Proc. 53, 305–312 (1993).
Van Steenkiste, N., Locke, S. A., Castelin, M., Marcogliese, D. J. & Abbott, C. L. New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes). Mol. Ecol. Resour. 15(4), 945–952. https://doi.org/10.1111/1755-0998.12358 (2015).
Khalifa, M. M. et al. Dogs as a source for the spreading of enteric parasites including zoonotic ones in Giza Province, Egypt. Res. Vet. Sci. 161, 122–131 (2023).
Ramadan, R. M., Khalifa, M. M., Kamel, N. O., Abdel-Wahab, A. M. & El-Bahy, M. M. The use of Giardia immunogenic protein fraction to distinguish assemblages in humans and animals. World’s Vet. J. 10(3), 421–428. https://doi.org/10.36380/scil.2020.wvj52 (2020).
Suvarna, K. S., Layton, C. & Bancroft, J. D. Bancroft’s Theory and Practice of Histological Techniques (Elsevier health sciences, 2018).
Ramadan, R. M., Khalifa, M. M., El-Akkad, D. M., Abdel-Wahab, A. M. & El-Bahy, M. M. Animal hydatid cyst genotypes as a potential substitute for human hydatid cyst as a source of antigen for diagnosis of zoonotic hydatidosis. J. Parasit. Dis. 45, 424–434. https://doi.org/10.1007/s12639-020-01309-2 (2021).
Khalifa, M. M. et al. Trichinocidal activity of a novel formulation of curcumin-olive oil nanocomposite in vitro. Vet. Parasitol. Reg. Stud. Rep. 41(100880), 1–9. https://doi.org/10.1016/j.vprsr.2023.100880 (2023).
Hussein, S. & Rezk, H. Macro and microscopic characteristics of the gastrointestinal tract of the Cattle Egret (Bubulcus ibis). Int. J. Anat. Res. 4(2), 2162–2174. https://doi.org/10.16965/IJAR.2016.169 (2016).
Kharoo, V. K. A review of the history and classification of the family Renicolidae (Dollfus, 1939), (Trematoda: Digenea). Indian J. Fund. Appl. Life Sci. 3, 6–12 (2013).
El-Naffar, M. K., Khalifa, R. M. & Sakla, A. A. Parasitofauna of the Egyptian aquatic birds. II. Trematode parasites of the giant heron (Ardea goliath) in Assiut governorate. J. Egypt. Soc. Parasitol. 10(1), 107–116 (1980).
Hassan, M. G. & Abd-El Aal, A. A. Studies on helminth parasites infesting some wild birds in Suez Canal area and Sinai Peninsula. Assiut Vet. Med. J. 40(80), 103–118. https://doi.org/10.21608/AVMJ.1999.182328 (1999).
Hayunga, E. G. Morphological adaptations of intestinal helminths. J. Parasitol. https://doi.org/10.2307/3282734 (1991).
Galaktionov, K. V., Solovyeva, A. I., Blakeslee, A. M. & Skírnisson, K. Overview of renicolid digeneans (Digenea, Renicolidae) from marine gulls of northern Holarctic with remarks on their species statuses, phylogeny and phylogeography. Parasitology 150(1), 55–77. https://doi.org/10.1017/S0031182022001500 (2023).
Cuartas, J. H., Alzate, J. F., Moreno-Herrera, C. X. & Marquez, E. J. Metagenomic analysis of orange colored protrusions from the muscle of Queen Conch Lobatus gigas (Linnaeus, 1758). Peer J. 6, 1–20. https://doi.org/10.7717/peerj.4307 (2018).
Flowers, J. R., Poore, M. F., Mullen, J. E. & Levy, M. G. Digeneans collected from piscivorous birds in North Carolina, USA. Comp. Parasitol. 71, 243–244 (2004).
Locke, S. A., Drago, F. B., Núñez, V., Souza, G. T. R. & Takemoto, R. M. Phylogenetic position of Diplostomum spp. from New World herons based on complete mitogenomes, rDNA operons, and DNA barcodes, including a new species with partially elucidated life cycle. Parasitol. Res. 119, 2129–2137. https://doi.org/10.1007/s00436-020-06713-4 (2020).
Locke, S. A., McLaughlin, J. D., Lapierre, A. R., Johnson, P. T. & Marcogliese, D. J. Linking larvae and adults of Apharyngostrigea cornu, Hysteromorpha triloba and Alaria mustelae (Diplostomoidea: Digenea) using molecular data. J. Parasitol. 97, 846–851. https://doi.org/10.1645/GE-2775.1 (2011).
Horne, E. C., Bray, R. A. & Bousfield, B. The presence of the trematodes Cardiocephaloides physalis and Renicola sloanei in the African Penguin Spheniscus demersus on the east coast of South Africa. Ostrich 82(2), 157–160. https://doi.org/10.2989/00306525.2011.603484 (2011).
Acknowledgements
The authors would like to acknowledge the support of farmers at Abou Rewash region, Giza, Egypt for collecting freshly dead Cattle Egret (Bubulcus ibis) and supplying it to the Parasitology Laboratory, Faculty of Veterinary Medicine.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
M.A.S. and O.A.M., R.M.R. designed the study, analyzed and interpreted the data. R.M.R., M.A.S. and O.A.M. undergo collection of samples, morphological and molecular identification. M.S. performed histopathological findings. All the authors acquired the data and revised the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salem, M.A., Mahdy, O.A., Shaalan, M. et al. The phylogenetic position and analysis of Renicola and Apharyngostrigea species isolated from Cattle Egret (Bubulcus ibis). Sci Rep 13, 16195 (2023). https://doi.org/10.1038/s41598-023-43479-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-43479-y
This article is cited by
-
A multidisciplinary study on Clinostomum infections in Nile tilapia: micro-morphology, oxidative stress, immunology, and histopathology
BMC Veterinary Research (2024)
-
Efficacy of silver nanoparticles against Trichinella spiralis in mice and the role of multivitamin in alleviating its toxicity
Scientific Reports (2024)
-
Morphological and molecular characterization of Linguatula serrata and evaluation of the health status of the infested dogs
Comparative Clinical Pathology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.